The ongoing pandemic has initiated a race for a cure among the biggest pharmaceutical companies in the world. This race has yielded fruits and vaccines have been rolled out to those in need. However, there’s still some hesitancy when it comes to the entire process, especially with the whole idea of injecting a needle into your body. Well, that could soon end.
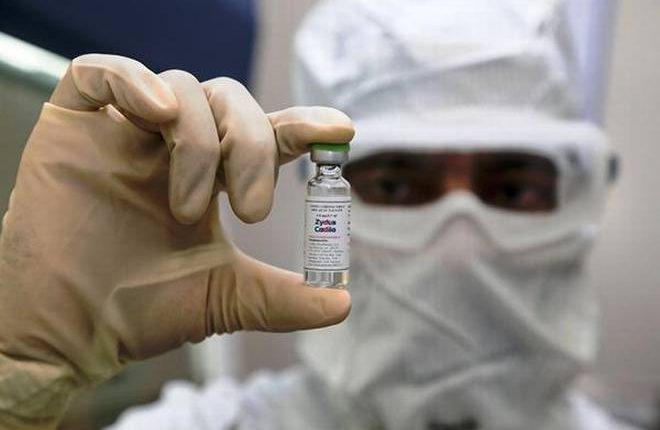
Yes, you read that right. ZyCOV-D, the world’s first needle-free, plasmid DNA vaccine is coming soon. Developed by Indian pharmaceutical firm Zydus Cadila, the three-dose vaccine has finally received the Emergency Use Authorization (EUA) from the Drug Controller General of India (DCGI). The second and third doses of this vaccine can be taken after 28 and 56 days respectively after the first dose.
How does a needle-free vaccine work? Needle-free injectors deliver the vaccine using a narrow stream of fluid to penetrate the skin and deliver the vaccine to the proper tissue depth. This leads to a lesser risk of developing side effects as well as is useful for people with a fear of needles.
“This is a historic milestone with ZyCoV-D, a product of Indian innovation becoming the world’s first DNA vaccine being offered for human use and supporting the world’s largest immunization drive,” said Pankaj R Patel, Chairman, Cadila Healthcare Ltd (Zydus Cadila). The vaccine is developed in partnership with India’s Department of Biotechnology (DBT), under its ‘Mission Covid Suraksha’, and implemented by Biotechnology Industry Research Assistance Council (BIRAC).
Zydus, which had applied for the emergency use license on July 1, claims to have conducted the largest clinical trial for the vaccine in the country in over 50 centers so far. The vaccine is an intra-dermal one and is the first vaccine for adolescents in the age group of 12-18, as well as the second indigenous vaccine after Covaxin.
It seems to be promising. After exhibiting robust immunogenicity and tolerability and safety profile in the adaptive Phase I/II clinical trials, it showed a primary efficacy of 66.6% for symptomatic RT-PCR positive cases in the interim analysis of the late-stage phase-III clinical trials of over 28,000 volunteers. Additionally, it is surmised that it provides 100% efficacy for the moderate disease of COVID-19 after no such disease was observed in the vaccine arm after the administration of the third (and final) dose. It is also easy to transport and store to supply people in remote locations.
According to the company, it has invested ₹400-500 crore in the development of the vaccine, including the repurposing of an existing manufacturing plant where “we are now producing, and our new plant will be ready by the end of July. By August, we should have the capacity to make 10 million doses a month and by the end of this year, we would have made 50 million doses of ZyCoV-D,” Sharvil Patel, managing director of Zydus Cadila, had said earlier. Patel added that Zydus is investing in a new plant to manufacture about 10-12 crore doses in a year. For now, Zydus aims to produce nearly a crore doses by mid-August or September and five crore doses by December.
Additionally, the vaccine works against the Delta variant, as was evident in the trials carried out during the peak of the second wave. For now, the company also aims to seek approval for a two-dose regimen of the vaccine.
The Tech Portal is published by Blue Box Media Private Limited. Our investors have no influence over our reporting. Read our full Ownership and Funding Disclosure →