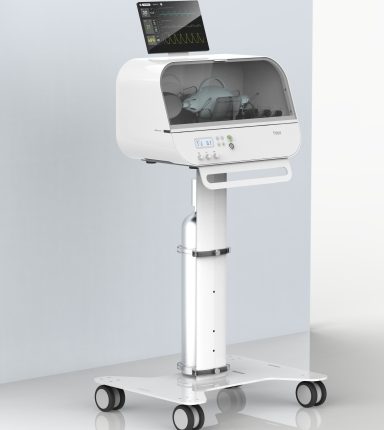
Fitbit isn’t exactly a medical company. Sure, the company’s bands are excellent tools to measure heartbeat and track your workouts, but that’s been the extent of the “medical” expertise the company has had. However, in light of the COVID-19 pandemic and the dastardly lack of medical facilities that followed, Fitbit has managed to secure approval from the Food and Drug Administration (FDA) for its Fitbit Flow emergency ventilator.
The ventilator that the company plans on delivering will be of the emergency resuscitator-style, which is to be used only when the proper hardware isn’t available. Resuscitator type ventilators replicate the function of the types of manual resuscitation bags used by paramedics and EMTs in the field.
“COVID-19 has challenged all of us to push the boundaries of innovation and creativity, and use everything at our disposal to more rapidly develop products that support patients and the health care systems caring for them,” said James Park, co-founder and CEO of Fitbit. “We saw an opportunity to rally our expertise in advanced sensor development, manufacturing, and our global supply chain to address the critical and ongoing need for ventilators and help make a difference in the global fight against this virus.”
The company says that it will produce ventilators that are high-quality, low-cost and easy-to-use. The Fitbit flow ventilator will be based upon the MIT E-Vent System, and specifications for Rapidly Manufactured Ventilation Systems. The company consulted with Oregon Health & Science University emergency medicine clinicians caring for COVID-19 patients at OHSU Hospital and worked with the Mass General Brigham Center for COVID Innovation working group on the design to meet the needs of practitioners.
The company is currently producing millions of Fitbit devices, and boasts vast infrastructure and manufacturing capabilities. It also has skilled workforce already working with sensors, and health monitoring. Fitbit says that with its past expertise, it can build ventilators that are on par with those being used by paramedics, all equipped with sophisticated instruments, sensors, and alarms that work together to support automated compressions and patient monitoring.
The device is supposed to be fairly easy to use. However, Fitbit does not aim for Fitbit Flow ventilators to replace the existing ones. These ventilators are just supposed to be a low cost, emergency substitute for the “real” ones, and not permanent replacements.
Fitbit is also in talks with state and federal agencies to understand the current need of ventilators and other emergency equipment in more detail. The company also plans to work with U.S. government and other organisations to address these needs in the very near future.